HOME > List of Voice of grantees > Development of an Artificial Nucleic Acid for Medical Applications
Development of an Artificial Nucleic Acid for Medical Applications"Creation of Industrial Infrastructure" 3rd Grant Period: April 2012–March 2015
Details of research conducted during the grant period
In simple terms, "developing artificial DNA with a simplified structure"
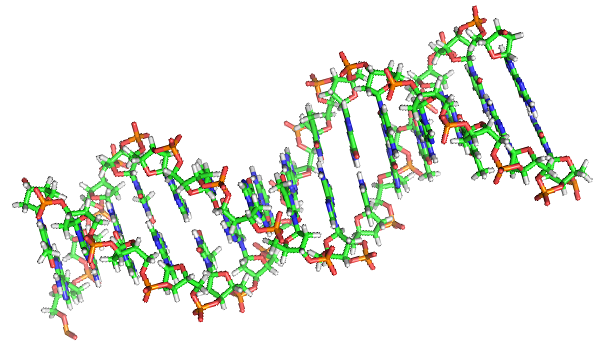 Double helix structure of DNA
Double helix structure of DNAWhat is DNA? Possessed by all life on earth, DNA is a macromolecule containing the genetic code for life. This structure, which appears in the accompanying illustration and which can be found in a textbook or other information source, comprises two chains of the double helix linked via a hydrogen bond. However, I broke this structure at a certain point. By accident, I discovered a structure that is even simpler and results in double chains that are more stable than those of natural DNA.
Natural DNA has no function by itself. However, artificial DNA developed with various functions (such as response to light or fluorescence emission) makes possible an extremely wide range of applications. The two major purposes of this research were as follows: to develop new functions, and to determine the science of why DNA is formed into a double helix structure with ribose*1 frames.
During the grant period of the Canon Foundation, I succeeded in creating DNA with flexible functions by incorporating a cartridge-type artificial nucleic acid in an appropriate position in the DNA. This was a great success that led to industrial applications, which is one of the Canon Foundation's objectives.
Because the cartridge approach is a major feature, the artificial nucleic acid "Threoninol-Nucleotide" I created is extremely advantageous in terms of cost and practical applications in contrast to conventional artificial nucleic acids. Typically, incorporating functional molecules in DNA requires at least seven steps of multistage synthesis. However, Threoninol-Nucleotide can be synthesized simply in three steps, making it possible to incorporate functional molecules in the desired position of the DNA.
Thus, it has become possible to create artificial DNA with a more simplified structure.
- *1
- Ribose is a type of sugar; specifically, it is a pentose monosaccharide. Ribose is formed into a nucleoside in combination with a nucleic acidic base, which is known as constituent sugar of ribonucleic acid. (Citation translated from the Japanese-language edition of Wikipedia)
Progress and development following the research funding period
Applications for personalized medical treatment and nucleic acidic medicine
Artificial nucleic acid is applicable to medicine. "Gene expression" means that gene information is copied from DNA to RNA, and the copied information is translated to become protein. For example, in the case of a gene related to disease, the simplest method of curing the disease is to target the RNA. Toward this end, however, even if natural DNA is incorporated into the body, it will be decomposed by enzymes; artificial DNA, on the other hand, cannot be decomposed by enzymes. The most important issue in nucleic acidic medicine is resistance to enzymes. Our artificial nucleic acid is absolutely enzyme-resistant and non-toxic.

For example, suppressing certain functions of RNA such as carcinogenesis results in cancer treatment. As a means of disabling the expression of virus RNA, the relevant RNA should be destroyed. Our approach provides high quality while reducing cost and the number of preparatory steps, resulting in a fast, low-cost, and high-quality process. The infrastructural technology of nucleic acidic medicine developed significantly during the period of the Canon Foundation's funding, in contrast to the time before the grant period.
Moreover, in the area of joint research, we have developed a method for direct observation of RNA expression in the cell; called the "fluorescence probe,*2 it enables us to detect specific RNA, which is helpful in diagnosis. Diagnostic medicine in this area will soon become commercially available.
- *2
- A general term for a functional molecule that reacts with specific molecules to cause a change in molecular structure; for example, it emits a strong fluorescent light or a change in the fluorescent color. This can results in selective fluorescent emissions from only cancer cells and visualization of the state and functions of biomolecules in vivo. (Citation translated from the Japanese-language edition of Wikipedia)
Were there any interesting incidents after your research began?
The most important factors related to research are that we must remain flexible and that we must never give up.
We failed several times when attempting artificial nucleic acid synthesis, but when we tried to use a chemical compound that was at hand (as a desperate measure), we finally succeeded at synthesis. This method is not based on the conventional approach, but on the "wrong" approach. After much trial and error, I was happy to have achieved success. For research, it is essential that we never give up. In addition, flexibility is key. Through all the repeated failures, I would frequently change my approach without being bound by conventional wisdom, and this is what eventually resulted in my success. Now, my approach is called the "Asanuma Method." Regardless of whether things go well or not, we must push forward with purpose; that is science. As long as we remember this, we will never reach a dead end.
What made you want to become a researcher?

I just set out to get a regular job after graduating from university. But because I had always been drawn to all scientific subjects through primary school and junior high school, I had a chance to meet some good teachers.
My professors at university were so busy that they weren't able to give me much guidance, but this was good for me because I was able to consider and overcome problems freely on my own. When research does not progress as expected, it's actually interesting, which is one of the pleasures of study.
After graduation, I was employed by Fujifilm Corporation, and then I had a chance to return to university as a researcher engaged in the study of DNA.
Why did you go back to university?
My teacher recommended me for a post as research assistant. When I worked at Fujifilm, I worked in optical science, so when I first examined DNA at the university, it appeared to be an aggregation of pigments. I am very fond of pigments, and I thought it was interesting that pigment could be a substitute for a base. So, I decided to try incorporating pigment into the DNA, and thus I started my DNA-related research.
When I worked at a company, I did not develop any product on my own, but I did manage to develop one after returning to university. My good fortune has been enjoyable.
What are your dreams for the future?
I have a strong desire to develop a domestic product in the market for nucleic acid medicine. Japanese pharmaceutical manufacturers have not yet succeeded in developing such a nucleic acid medicine. The level of research on nucleic acid in Japan is quite high, but no products have yet been commercialized. My goal is to commercialize a nucleic acid medicine. The next is to reveal the science behind the double helix structure of DNA created by God. The structure of DNA is in response to the existing global environment; so, if the environment were different, the structure might be different. In light of this, I have wondered why DNA has adopted the current structure through several hundred million years of exposure to this environment. It may be the result of an accident, but I think it has a certain meaning. As a chemist, I hope to continue pursuing the deeper meaning from now on.
Profile
Hiroyuki Asanuma
Professor, Nagoya University Graduate School of Engineering, Specialist in Biomolecular Engineering, Doctor of Engineering






